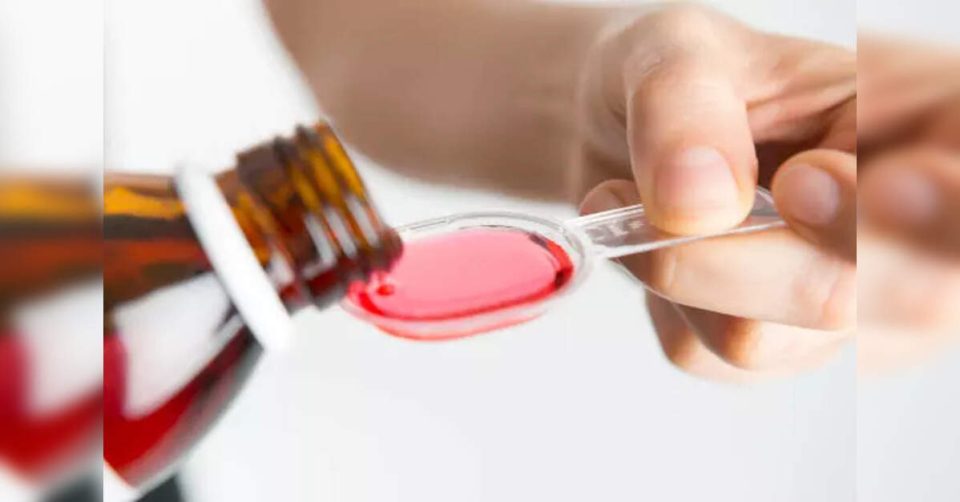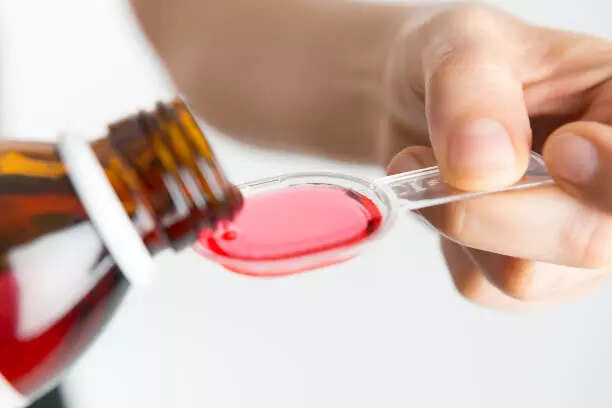
New Delhi: The State Drug Testing Laboratory of Tamil Nadu has detected diethylene glycol (DEG) beyond permissible limits in samples of the cough syrup allegedly linked to the deaths of nine children in Madhya Pradesh’s Chhindwara district.
The samples were collected from the drugmaker—Kanchipuram based manufacturing premises, following a request from the Madhya Pradesh government.
Notably a day before this announcement samples of the syrup tested by CDSCO and the MP state drug regulator were “found to be free of DEG/EG” contaminants.
Giving the clarification on the stand the Union Health ministry in a statement noted that, the results of testing of these samples (those by the TN FDA) were shared with us late evening, yesterday i.e. October 3 2025.
“A multidisciplinary team comprising of experts from NIV, ICMR, NEERI, CDSCO and AIIMS, Nagpur is still analysing the various samples and factors to assess the cause of deaths,” it added.
Coldrif cough syrup, manufactured by Tamilnadu-based Sresan Pharma, is suspected to have caused kidney failure leading to the deaths of at least nine children in Madhya Pradesh’s Chhindwara district over the past month.
Following the announcement, Madhya Pradesh, Chief Minister Mohan Yadav in a post on X wrote that, “the deaths of children in Chhindwara due to Coldrif syrup are extremely tragic and a team has also been formed at the state level to investigate this matter.” (this is an auto-translated version, as the original post was published in a different language.)
“The sale of this syrup (Coldrif) has been banned across the entire Madhya Pradesh and a ban is also being imposed on the sale of other products from the company that manufactures the syrup,” he added.
According to several e-pharmacy platforms, Coldrif syrup is prescribed to children with symptoms of cough and cold, and the drug active ingredients includes Chlorpheniramine Maleate, Paracetamol and Phenylephrine.